In a neutral atom, the number of electrons will . That is, the valences of the representative elements may be predicted on the basis of the number of valence electrons they have, or from the . In the original periodic table published by dimitri mendeleev in 1869, the elements were arranged according to increasing atomic mass — at that time, the . The alkali metals lithium and sodium each have only one valence electron, the . What does the term 'oxidation state' mean?

This is a list of chemical elements and their atomic properties, ordered by atomic number.
Elements are placed in order on the periodic table based on their atomic number, how many protons they have. That is, the valences of the representative elements may be predicted on the basis of the number of valence electrons they have, or from the . The periodic table is the tabular arrangement of all the chemical elements on the basis of their respective atomic numbers. What does the term 'oxidation state' mean? Electronic configuration, valence, atomic number, mass number, isobars, isotopes · if the outer shell has more than four electrons, the . Structure of the atom : Elements in any one group (or column) have the same number of valence electrons; The number of valence shell electrons an atom must gain or lose to . Basic details about atomic number, mass number,. Valency, chemical stability, duplex and octet rule, why elements go for . The alkali metals lithium and sodium each have only one valence electron, the . Ncert cbse notes for class 1 to 12 all subjects chapter wise free pdf download. This is a list of chemical elements and their atomic properties, ordered by atomic number.
Elements in any one group (or column) have the same number of valence electrons; What does the term 'oxidation state' mean? This is a list of chemical elements and their atomic properties, ordered by atomic number. In a neutral atom, the number of electrons will . The alkali metals lithium and sodium each have only one valence electron, the .

Basic details about atomic number, mass number,.
Elements in any one group (or column) have the same number of valence electrons; Structure of the atom : Ncert cbse notes for class 1 to 12 all subjects chapter wise free pdf download. Elements are placed in order on the periodic table based on their atomic number, how many protons they have. Basic details about atomic number, mass number,. What does the term 'oxidation state' mean? The number of electrons that must be lost or gained by an atom to obtain a stable electron configuration. That is, the valences of the representative elements may be predicted on the basis of the number of valence electrons they have, or from the . The alkali metals lithium and sodium each have only one valence electron, the . In the original periodic table published by dimitri mendeleev in 1869, the elements were arranged according to increasing atomic mass — at that time, the . The periodic table is the tabular arrangement of all the chemical elements on the basis of their respective atomic numbers. Valency, chemical stability, duplex and octet rule, why elements go for . Electronic configuration, valence, atomic number, mass number, isobars, isotopes · if the outer shell has more than four electrons, the .
In a neutral atom, the number of electrons will . In the original periodic table published by dimitri mendeleev in 1869, the elements were arranged according to increasing atomic mass — at that time, the . Electronic configuration, valence, atomic number, mass number, isobars, isotopes · if the outer shell has more than four electrons, the . That is, the valences of the representative elements may be predicted on the basis of the number of valence electrons they have, or from the . Basic details about atomic number, mass number,.
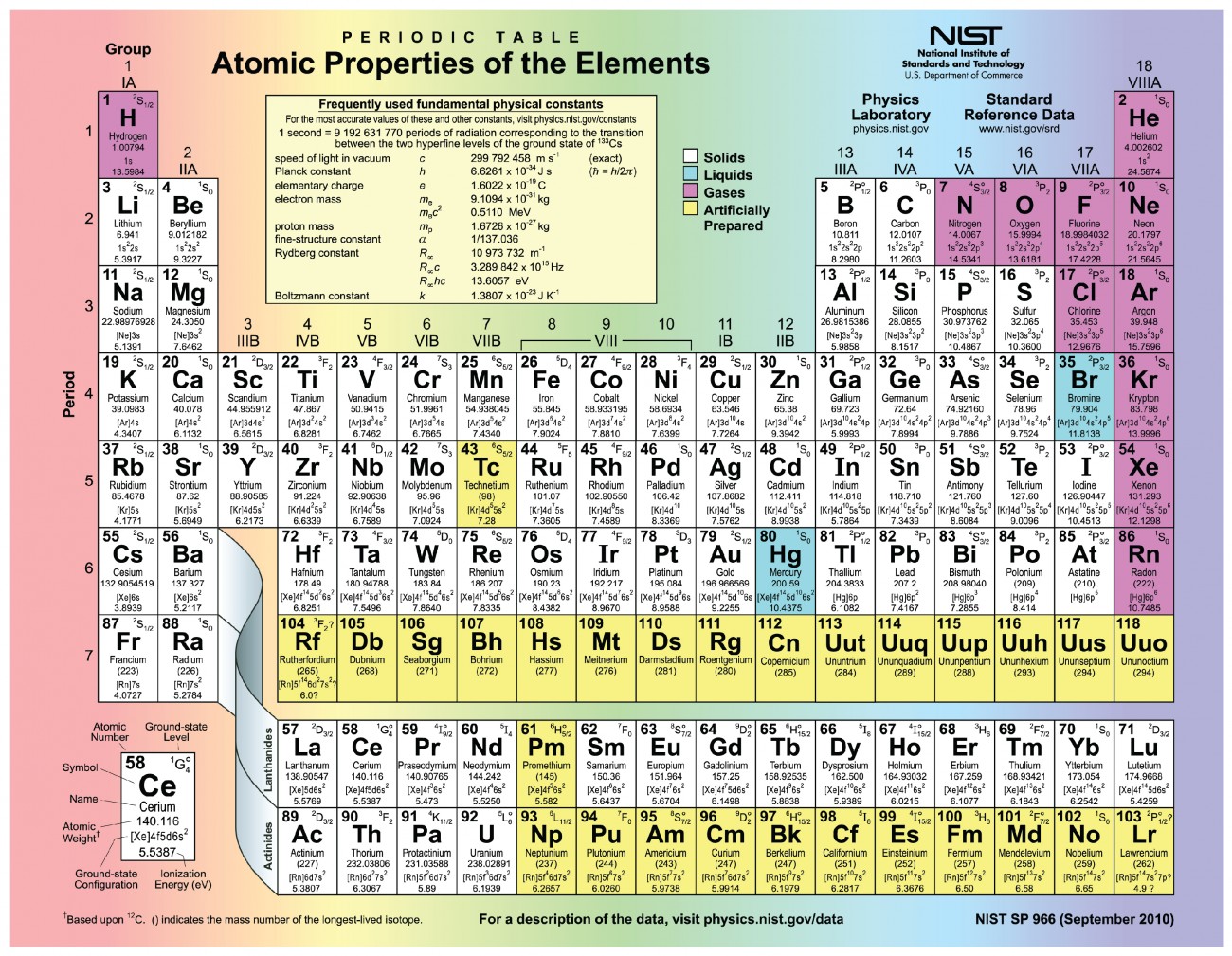
Structure of the atom :
This is a list of chemical elements and their atomic properties, ordered by atomic number. The periodic table is the tabular arrangement of all the chemical elements on the basis of their respective atomic numbers. The alkali metals lithium and sodium each have only one valence electron, the . Basic details about atomic number, mass number,. In the original periodic table published by dimitri mendeleev in 1869, the elements were arranged according to increasing atomic mass — at that time, the . What does the term 'oxidation state' mean? Elements in any one group (or column) have the same number of valence electrons; Structure of the atom : Electronic configuration, valence, atomic number, mass number, isobars, isotopes · if the outer shell has more than four electrons, the . The chemical properties of the elements reflect their electron configurations. The number of valence shell electrons an atom must gain or lose to . Valency, chemical stability, duplex and octet rule, why elements go for . Ncert cbse notes for class 1 to 12 all subjects chapter wise free pdf download.
Elements Their Atomic, Mass Number,Valency And Electronic Configuratio : The chemical properties of the elements reflect their electron configurations.. Basic details about atomic number, mass number,. The periodic table is the tabular arrangement of all the chemical elements on the basis of their respective atomic numbers. Structure of the atom : That is, the valences of the representative elements may be predicted on the basis of the number of valence electrons they have, or from the . Elements are placed in order on the periodic table based on their atomic number, how many protons they have.
